
Chemistry, 19.11.2020 05:40 SmartKitty
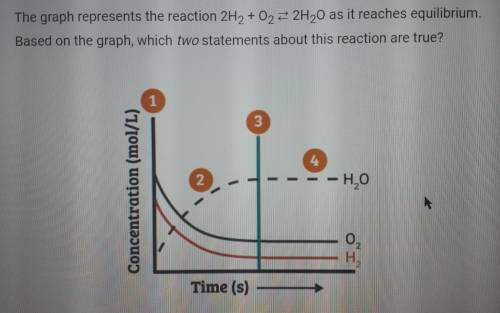
The graph represents the reaction 2H2 +02 = 2H20 as it reaches equilibrium. Based on the graph, which two statements about this reaction are true?
A. At point 2, the concentrations of H20, 02, and H2 are all changing toward their equilibrium concentrations.
B. The rate of formation of products is equal to the rate of formation of reactants only after point 4.
C. After point 2, the rate of formation of products is equal to the rate of formation of reactants.
D. At point 1, more reactants are converted to products than products are converted to reactants.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Each pair of clay balls represents to planetesimals if each plane test molluscum pound of the same material and is separated by the same distance which pair experiences the greatest gravitational attraction
Answers: 2

Chemistry, 22.06.2019 05:30
The climate of the continental united states is generally 1. tropical 2. temperate 3. arctic 4. highland
Answers: 1

Chemistry, 22.06.2019 10:30
Geothermal energy for industrial use is available almost anywhere. a.true b.false
Answers: 2

Chemistry, 22.06.2019 16:30
Correct relationship between molecular formula and empirical formula
Answers: 1
You know the right answer?
The graph represents the reaction 2H2 +02 = 2H20 as it reaches equilibrium. Based on the graph, whic...
Questions







Computers and Technology, 23.10.2019 02:00








Mathematics, 23.10.2019 02:00







