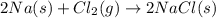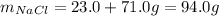Chemistry, 19.11.2020 03:30 seanholmes91405
A chemistry student combines 23.0 grams of sodium and 71.0 grams of chloride to form sodium chloride. According to the law of conservation of mass, what mass of sodium chloride should be formed if the reaction proceeds to completion?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Joe shines white light into a bowl half full of water at an angle of incident of 27.5°. calculate the angle of refraction in the water given the indices of refraction for air and water are 1.00 and 1.36, respectively.
Answers: 2


Chemistry, 22.06.2019 16:30
Explain in detail of the four major scientific developments that spurred the formulation of the plate tectonics theory
Answers: 2

Chemistry, 22.06.2019 17:00
Astable electron arrangement for an atom is one that does not easily change. how is this arrangement arrived at? a. valence electrons are transferred or shared to create a full outer shell of electrons. b. valence electrons are discarded into space to create a full outer shell of electrons. c. protons (positive charge) pair with valence electrons (negative charge) to create a strong bond. d. outer shells with valence electrons are transferred or shared.
Answers: 2
You know the right answer?
A chemistry student combines 23.0 grams of sodium and 71.0 grams of chloride to form sodium chloride...
Questions







German, 11.03.2021 19:40

Mathematics, 11.03.2021 19:40




Mathematics, 11.03.2021 19:40



Health, 11.03.2021 19:40

Mathematics, 11.03.2021 19:40

History, 11.03.2021 19:40


Mathematics, 11.03.2021 19:40

Chemistry, 11.03.2021 19:40