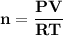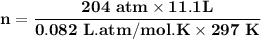Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 01:30
What is the value of keq for the reaction expressed in scientific notation
Answers: 1

Chemistry, 22.06.2019 20:20
Nitric acid can be formed in two steps from the atmospheric gases nitrogen and oxygen, plus hydrogen prepared by reforming natural gas. in the first step, nitrogen and hydrogen react to form ammonia: (g) (g) (g) in the second step, ammonia and oxygen react to form nitric acid and water: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of nitric acid from nitrogen, hydrogen and oxygen from these reactions. round your answer to the nearest .
Answers: 3

Chemistry, 22.06.2019 22:30
Which process describes vaporization that takes place below the surface of a liquid? condensation melting boiling evaporation
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
A steel cylinder for scuba diving contains 11.1 L of compressed air. The pressure inside the cylinde...
Questions


Mathematics, 16.06.2021 20:30

Arts, 16.06.2021 20:30

Mathematics, 16.06.2021 20:30

Mathematics, 16.06.2021 20:30


Law, 16.06.2021 20:30

Mathematics, 16.06.2021 20:30


Mathematics, 16.06.2021 20:30


Mathematics, 16.06.2021 20:30




Mathematics, 16.06.2021 20:40

Mathematics, 16.06.2021 20:40

Mathematics, 16.06.2021 20:40

Chemistry, 16.06.2021 20:40

Mathematics, 16.06.2021 20:40