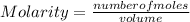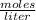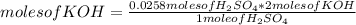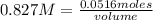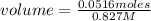Chemistry, 18.11.2020 17:10 ahmedeldyame
In a titration of 35.00 mL of 0.737 M H2SO4, mL of a 0.827 M KOH solution is required for neutralization.
A) 35.0
B) 1.12
C) 25.8
D) 62.4
E) 39.3

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Butadiene undergoes a reaction at a certain temperature in the gas phase as follows: 2c4h6(g) --> c8h12(g) the following data were collected for this reaction: time (min) [c4h6] (m) 0 0.36 15 0.30 30 0.25 48 0.19 75 0. determine the order of the reaction and the rate constant. 1st order and k = 4.3x10 -4 s-1 1st order and k = 2.3x10-4 s-1 2nd order and k = 4.3x10-4 s-1 2nd order and k = 2.3x10-4 s-1 zero and k = 4.3x10-4 s-1
Answers: 3

Chemistry, 22.06.2019 09:20
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 12:00
What is a possible quantum number set for an electron in the 3s orbital of a magnesium atom
Answers: 1

You know the right answer?
In a titration of 35.00 mL of 0.737 M H2SO4, mL of a 0.827 M KOH solution is required for neutraliz...
Questions


Mathematics, 08.10.2019 22:00



History, 08.10.2019 22:00










Mathematics, 08.10.2019 22:00




Physics, 08.10.2019 22:00