

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:30
Describe the interaction that occurs between two objects with the same electrical charge.
Answers: 1

Chemistry, 22.06.2019 10:30
Skills of homo sapiens were found an excavation. the skulls were preserved because the bodies were frozen. so, these fossils are (blank) fossils.the image shows the evolution of skulls beginning 2 to 3 million years ago. based on the image, modern human skulls(blank) ape skulls.
Answers: 1

Chemistry, 22.06.2019 19:00
Suppose that a certain fortunate person has a net worth of $71.0 billion ($7.10×1010). if her stock has a good year and gains $3.20 billion (3.20×109) in value, what is her new net worth?
Answers: 3

You know the right answer?
For the equilibrium that exists in an aqueous solution of nitrous acid (HNO2, a weak acid), the equi...
Questions

Computers and Technology, 17.04.2020 20:02


Mathematics, 17.04.2020 20:02

Mathematics, 17.04.2020 20:02

Mathematics, 17.04.2020 20:02

Mathematics, 17.04.2020 20:02




Computers and Technology, 17.04.2020 20:02




Mathematics, 17.04.2020 20:02


Mathematics, 17.04.2020 20:02

Mathematics, 17.04.2020 20:02



History, 17.04.2020 20:02

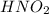
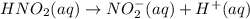
![k_a = \frac{[NO_{2}^{-}][H^{+}]}{HNO_{2}}](/tpl/images/0908/7640/67909.png)


