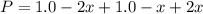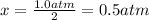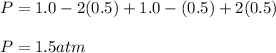Chemistry, 18.11.2020 17:10 juliah6925
Consider the generic reaction: 2 A(g) + B(g) → 2 C(g). If a flask initially contains 1.0 atm of A and 1.0 atm of B, what is the pressure in the flask if the reaction proceeds to completion? (Assume constant volume and temperature.)a. 1.0 atmb. 1.5 atmc. 2.0 atmd. 3.0 atm

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Acar tire has a pressure of 2.38 atm at 15.2 c. if the pressure inside reached 4.08 atm, the tire will explode. how hot would the tire have to get for this to happen? report the temperature in degrees celsius.
Answers: 2

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2

You know the right answer?
Consider the generic reaction: 2 A(g) + B(g) → 2 C(g). If a flask initially contains 1.0 atm of A an...
Questions




Mathematics, 16.04.2020 20:12




Mathematics, 16.04.2020 20:12




Mathematics, 16.04.2020 20:12

Social Studies, 16.04.2020 20:12

Mathematics, 16.04.2020 20:12

Mathematics, 16.04.2020 20:12




History, 16.04.2020 20:13

History, 16.04.2020 20:13

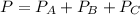
 we can write:
we can write: