1.2 x 10²³atoms
Explanation:
Given parameters:
Mass of CO(NH₂) = 12g
Unknown:
Number of Nitrogen atoms = ?
Solution:
Given compound;
CO(NH₂);
In;
1 mole of CO(NH₂), we have 1 mole of N
let us find the number of moles of CO(NH₂);
Number of moles = 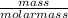
Number of moles = 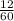 = 0.2moles
= 0.2moles
Since
1 mole of CO(NH₂), we have 1 mole of N
0.2 mole of CO(NH₂) will have 0.2mole of N
1 mole of a substance contains 6.02 x 10²³ atoms
0.2 mole of N will contain 0.2 x 6.02 x 10²³ atoms = 1.2 x 10²³atoms



























