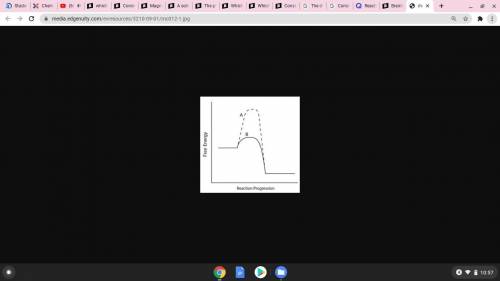
Consider the energy diagram below.
Which line indicates a higher reaction rate?
A because it...


Answers: 1


Another question on Chemistry




Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1
You know the right answer?
Questions



Geography, 07.05.2020 11:01


History, 07.05.2020 11:01

Mathematics, 07.05.2020 11:01

Mathematics, 07.05.2020 11:01




Social Studies, 07.05.2020 11:01



English, 07.05.2020 11:01


English, 07.05.2020 11:01