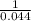19. The reaction 2NOBr (g) → 2 NO (g) + Br2 (g) is a second-order reaction with a rate constant of 0.80 M-1s-1 at 11 °C. If the initial concentration of NOBr is 0.0440 M, the concentration of NOBr after 6.0 seconds is . A) 0.0276 M B) 0.0324 M C) 0.0363 M D) 0.0348 M E) 0.0402 M

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 16:00
As changes in energy levels of electrons increase, the frequencies of atomic line spectra they emit
Answers: 2

Chemistry, 22.06.2019 22:30
What relationship exists between an enzyme and a catalyst?
Answers: 1

Chemistry, 23.06.2019 08:40
Which statement is true according to the kinetic theory? a. molecules of different gases with the same mass and temperature always have the same average density. b. molecules of different gases with the same mass and temperature always have the same average volume. c. molecules of different gases with the same mass and temperature always have the same pressure. d. molecules of different gases with the same mass and temperature always have the same molecular mass. e. molecules of different gases with the same mass and temperature always have the same average kinetic energy.
Answers: 1
You know the right answer?
19. The reaction 2NOBr (g) → 2 NO (g) + Br2 (g) is a second-order reaction with a rate constant of 0...
Questions

Mathematics, 19.09.2019 10:50



Business, 19.09.2019 10:50




Mathematics, 19.09.2019 10:50

History, 19.09.2019 10:50

Mathematics, 19.09.2019 10:50


Chemistry, 19.09.2019 10:50

History, 19.09.2019 10:50

Spanish, 19.09.2019 10:50


Mathematics, 19.09.2019 10:50




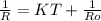
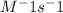
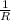 = 0.8*6+
= 0.8*6+