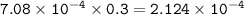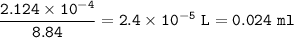Chemistry, 17.11.2020 06:30 jrfranckowiak
What volume (in mL) of 8.84 M HBr would be required to make 300.0 mL of a solution with a pH of 3.15?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 19:00
The skeletal system performs a variety of functions that are crucial to maintaining life processes. what function is performed in the bone marrow, but not in the ossified bones of the skeleton? a oxygen transportation c mineral storage b. muscle attachment d red blood cell production
Answers: 3

Chemistry, 22.06.2019 02:30
What is the relation between concentration of reactants and the rate of chemical reaction?
Answers: 1


Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2
You know the right answer?
What volume (in mL) of 8.84 M HBr would be required to make 300.0 mL of a solution with a pH of 3.15...
Questions

History, 19.01.2021 18:40





Health, 19.01.2021 18:40


Chemistry, 19.01.2021 18:40

Computers and Technology, 19.01.2021 18:40

Geography, 19.01.2021 18:40


History, 19.01.2021 18:40


Social Studies, 19.01.2021 18:40


Social Studies, 19.01.2021 18:40


Mathematics, 19.01.2021 18:40


![\tt [H^+]=10^{-3.15}=7.08\times 10^{-4}](/tpl/images/0903/9462/83b78.png)