
Chemistry, 17.11.2020 04:20 tinasidell1972
when 0.20 m nh4cl (aq) and 0.20 m naoh(aq) are mixed, the reaction represented by the equation above occurs and a strong smell of ammonia, nh3 is observed. based on this information, which of the following statements is true? And Why??
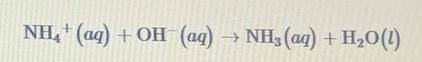


Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What must happen before a body cell can begin mitotic cell division
Answers: 1

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1

Chemistry, 22.06.2019 16:00
Sulfuric acid is a polyprotic acid. write balanced chemical equations for the sequence of reactions that sulfuric acid can undergo when it's dissolved in water.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
when 0.20 m nh4cl (aq) and 0.20 m naoh(aq) are mixed, the reaction represented by the equation above...
Questions




Biology, 08.10.2019 19:30

History, 08.10.2019 19:30

Mathematics, 08.10.2019 19:30

Biology, 08.10.2019 19:30

Mathematics, 08.10.2019 19:30



Biology, 08.10.2019 19:30

Mathematics, 08.10.2019 19:30



Social Studies, 08.10.2019 19:30




Mathematics, 08.10.2019 19:30



