
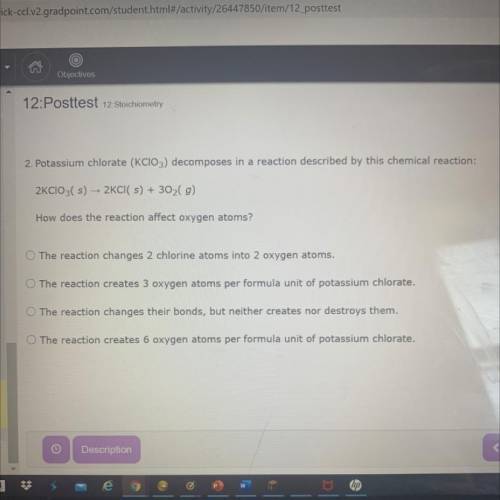
Potassium chlorate (KClO3) decomposes in a reaction described by this chemical reaction:
2KCIO3( s) - 2KCI( s) + 302(g)
How does the reaction affect oxygen atoms?
A. The reaction changes 2 chlorine atoms into 2 oxygen atoms.
B. The reaction creates 3 oxygen atoms per formula unit of potassium chlorate.
C. The reaction changes their bonds, but neither creates nor destroys them.
D. The reaction creates 6 oxygen atoms per formula unit of potassium chlorate.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 14:00
What is the ph of a solution that has a hydrogen ion concentration of 1.0 * 10 -9 m?
Answers: 2

Chemistry, 22.06.2019 22:30
Draw the aromatic compound toluene (methylbenzene). show all hydrogen atoms, including those on the ring.
Answers: 1

Chemistry, 22.06.2019 23:00
What element has similar physical and chemical properties as boron.
Answers: 1

Chemistry, 23.06.2019 03:30
In chemistry, the type of an atom (what element it is) is determined by: a) the number of protons it contains in its nucleus.b) the number of neutrons it contains in its nucleus.c) the number of protons it has in a cloud around the nucleus.d) the number of neutrons it has in a cloud around the nucleus.e) the number of electrons it exchanges with its neighbors.
Answers: 1
You know the right answer?
Potassium chlorate (KClO3) decomposes in a reaction described by this chemical reaction:
2KCIO3( s)...
Questions


Mathematics, 08.06.2020 05:57

Mathematics, 08.06.2020 05:57




Mathematics, 08.06.2020 05:57


Mathematics, 08.06.2020 05:57




Mathematics, 08.06.2020 05:57



Mathematics, 08.06.2020 06:57


History, 08.06.2020 06:57

Biology, 08.06.2020 06:57

Mathematics, 08.06.2020 06:57



