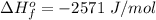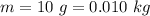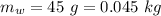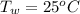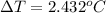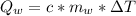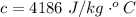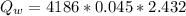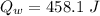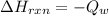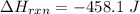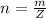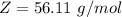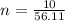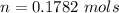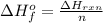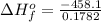Chemistry, 16.11.2020 17:30 batmanmarie2004
10g of Compound X with molecular formula are burned in a constant-pressure calorimeter containing 45g of water at 25. The temperature of the water is observed to rise by 2.432. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound at .

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:30
Find the empirical formula of each of the following compounds. given mass or for each element in a sample of the compound 3,611 g ca; 6.389 g c1
Answers: 1

Chemistry, 22.06.2019 14:50
Which of the following is most likely true about water in chemical solutions?
Answers: 1

Chemistry, 22.06.2019 18:00
Heat is the total potential energy of a substance that can be transferred. true false
Answers: 1

Chemistry, 22.06.2019 20:00
State one important difference between a physical change and a chemical change?
Answers: 1
You know the right answer?
10g of Compound X with molecular formula are burned in a constant-pressure calorimeter containing 45...
Questions

Mathematics, 11.11.2020 01:50

History, 11.11.2020 01:50

Mathematics, 11.11.2020 01:50

Arts, 11.11.2020 01:50




History, 11.11.2020 01:50

Mathematics, 11.11.2020 01:50



Mathematics, 11.11.2020 01:50

Biology, 11.11.2020 01:50

Mathematics, 11.11.2020 01:50


Mathematics, 11.11.2020 01:50

Mathematics, 11.11.2020 01:50


Mathematics, 11.11.2020 01:50

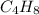 are burned in a constant-pressure calorimeter containing 45g of water at
are burned in a constant-pressure calorimeter containing 45g of water at 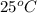 . The temperature of the water is observed to rise by 2.432. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound at
. The temperature of the water is observed to rise by 2.432. (You may assume all the heat released by the reaction is absorbed by the water, and none by the calorimeter itself.) Calculate the standard heat of formation of Compound at