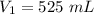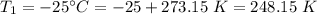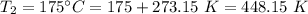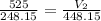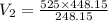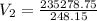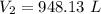Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2

Chemistry, 22.06.2019 15:30
A1.5l container holds p.50 grams of an unknown gas at a pressure of 0.44 atm and a temperature of 50.c what is the molar mass of the unknown gas
Answers: 1

Chemistry, 22.06.2019 17:30
Take a look at this dandelion. the yellow flower on the right is pollinated and the seeds on the left are transported by
Answers: 2

Chemistry, 22.06.2019 19:00
A4.86 g piece of metal was placed in a graduated cylinder containing 15.5 ml of water. the water level rose to 17.3 ml. what is the density of the metal. i need the steps of how to solve it to so i can use a formula to work out other problems.
Answers: 1
You know the right answer?
A gas with a volume of 525 mL at a temperature of -25°C is heated to 175°C.
What is the new volume,...
Questions




Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50



Social Studies, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50


Mathematics, 03.02.2021 16:50

History, 03.02.2021 16:50


Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

Mathematics, 03.02.2021 16:50

History, 03.02.2021 16:50


English, 03.02.2021 16:50

Chemistry, 03.02.2021 16:50

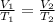
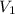 is the initial volume
is the initial volume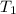 is the initial temperature
is the initial temperature 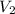 is the final volume
is the final volume 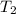 is the final temperature
is the final temperature