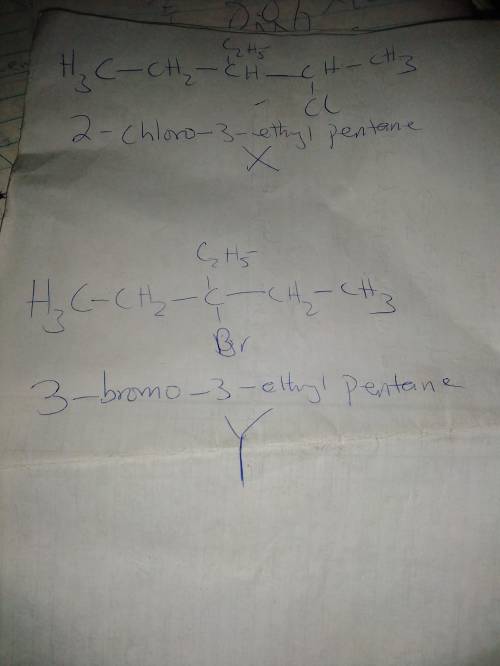Chemistry, 16.11.2020 16:50 crispingolfer1864
Compounds X has the formula C7H15Cl; Y is C7H15Br. X undergoes base-promoted E2 elimination to give a single alkene product Z. Y likewise reacts under similar conditions to give a single alkene product that is isomeric with Z Catalytic hydrogenation of Z affords 3-ethylpentane. X readily reacts in SN2 fashion with sodium iodide in acetone. Y does not undergo a similar SN2 reaction. Propose structures for X and Y.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:50
How does a scientist the government? a. the scientist tells people in society what to do. b. the scientist determines the policies that the government spends money on. c. the scientist provides unbiased information to the government. d. the scientist makes laws based on his or her research results.
Answers: 1


Chemistry, 22.06.2019 14:50
How are evaporation and sublimation similar? a both involve the formation of a gas. b both release energy to the surroundings. c both take place throughout a solid. d both take place at the surface of a liquid.
Answers: 1

Chemistry, 22.06.2019 19:00
Nan element’s square on the periodic table, the number with the greatest numerical value represents the
Answers: 3
You know the right answer?
Compounds X has the formula C7H15Cl; Y is C7H15Br. X undergoes base-promoted E2 elimination to give...
Questions

English, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30


Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30


Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30

Social Studies, 26.09.2019 01:30




Mathematics, 26.09.2019 01:30

Mathematics, 26.09.2019 01:30