Which arrow represents the change of state described
above?
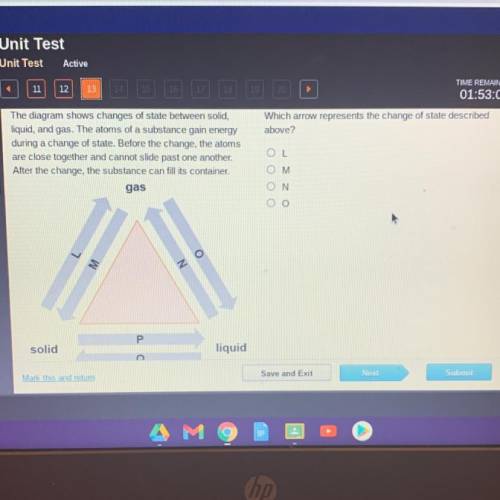
The diagram shows changes of stat...

Chemistry, 16.11.2020 04:30 deveronyarbrough
Which arrow represents the change of state described
above?
The diagram shows changes of state between solid,
liquid, and gas. The atoms of a substance gain energy
during a change of state. Before the change, the atoms
are close together and cannot slide past one another.
After the change, the substance can fill its container.
OL
OM
gas
ON
o
M
Z
Р
solid
liquid

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:30
Asample of ammonia reacts with oxygen as shown. 4nh3(g) + 5o2(g) 4no(g) + 6h2o(g) what is the limiting reactant if 4.0 g of nh3 react with 8.0 g of oxygen? o2 because it produces only 0.20 mol of no. nh3 because it produces only 0.20 mol of no. o2 because it produces two times less no than nh3. nh3 because it produces three times more no than o2.
Answers: 3

Chemistry, 22.06.2019 05:30
Match the following vocabulary terms to their definitions. 1. amount of energy required to change 1 gram of material from the solid to the liquid state at its melting point 2. a measure of the kinetic energy of the particles of a substance 3. the amount of heat energy required to raise the temperature of 1 gram of liquid water from 14.5°c to 15.5°c 4. amount of energy required to change 1 gram of material from the liquid to the gaseous state at its boiling point 5. the amount of energy required to change 1 gram of a substance 1°c a. temperature b. latent heat of vaporization c. latent heat of fusion d. calorie e. specific heat
Answers: 1


Chemistry, 22.06.2019 09:20
What will most likely happen when two bromine atoms bond together?
Answers: 3
You know the right answer?
Questions




Mathematics, 24.04.2020 19:44


Mathematics, 24.04.2020 19:44

Health, 24.04.2020 19:44

Mathematics, 24.04.2020 19:44


Business, 24.04.2020 19:44




Biology, 24.04.2020 19:44


Mathematics, 24.04.2020 19:44


Mathematics, 24.04.2020 19:44

Social Studies, 24.04.2020 19:44

History, 24.04.2020 19:45




