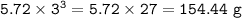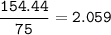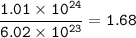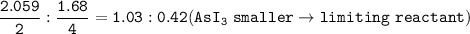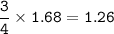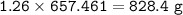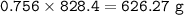The compound As2I4 is synthesized by reaction of arsenic metal with arsenic triiodide. If a solid cubic block of arsenic (d = 5.72 g/cm3 ) that is 3.00 cm on edge is allowed to react with 1.01 * 10^24 molecules of arsenic triiodide, how much As2I4 can be prepared? If the percent yield of As2I4 was 75.6%, what mass of As2I4 was actually isolated?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 13:30
Which statements are true concerning mineral formation? check all that apply. the slower the cooling, the larger the crystals. the faster the cooling, the smaller the crystals. crystals formed from magma are smaller than crystals formed from lava. minerals can only form in solutions when the solution is heated deep underground. when a solution cools, elements and compounds leave the solution and crystallize as minerals. minerals formed from hot water solutions can form narrow channels in the surrounding rock.
Answers: 1

Chemistry, 22.06.2019 16:00
Which of the following is the correct definition of chemical energy? a. energy an object has because of its motion or position b. energy resulting from the flow of charged particles, such as electrons or ions c. energy produced from the splitting of atoms d. energy stored in chemical bonds of molecules
Answers: 1

Chemistry, 22.06.2019 22:30
Write and balance the chemical equation that represents the reaction of aqueous sulfuric acid with aqueous sodium hydroxide to form water and sodium sulfate. include phases.
Answers: 1
You know the right answer?
The compound As2I4 is synthesized by reaction of arsenic metal with arsenic triiodide. If a solid cu...
Questions

Health, 18.12.2021 01:00

SAT, 18.12.2021 01:00

Geography, 18.12.2021 01:00

Mathematics, 18.12.2021 01:00


History, 18.12.2021 01:00

Mathematics, 18.12.2021 01:00

History, 18.12.2021 01:00


Social Studies, 18.12.2021 01:00

English, 18.12.2021 01:00

SAT, 18.12.2021 01:00





Biology, 18.12.2021 01:00



Chemistry, 18.12.2021 01:00