
Chemistry, 14.11.2020 14:00 briweaver9993
A 15.0 mL solution of Sr(OH)2 is neutralized with 38.5 mL of 0.350 M HCI. What is the concentration of the original Sr(OH)2 solution?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
How many atoms of mg are present in 97.22 grams of mg? 6.022 × 1023 2.408 × 1024 4.818 × 1024 5.855 × 1025
Answers: 3

Chemistry, 22.06.2019 17:40
If 3 moles of a compound use 24 j of energy in a reaction, what is the a hreaction in j/mol?
Answers: 1

Chemistry, 22.06.2019 21:30
How many liters of 3.0 m naoh solution will react with 0.60 liters of 4.0 m h2so4? h2so4 + naoh → na2so4 + h2o 1.2 l 1.6 l 2.4 l 2.8 l
Answers: 3

Chemistry, 23.06.2019 03:00
In which of the following phases of matter do molecules have the highest amount of energy? a. liquid b. gel c. solid d. gas
Answers: 2
You know the right answer?
A 15.0 mL solution of Sr(OH)2 is neutralized with 38.5 mL of 0.350 M
HCI. What is the concentration...
Questions



Mathematics, 31.01.2020 22:53

History, 31.01.2020 22:53



Mathematics, 31.01.2020 22:53

Mathematics, 31.01.2020 22:53


Mathematics, 31.01.2020 22:53

Mathematics, 31.01.2020 22:53


History, 31.01.2020 22:53

Mathematics, 31.01.2020 22:53

English, 31.01.2020 22:54





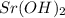 solution is 0.449 M and the further calculation can be defined as follows:
solution is 0.449 M and the further calculation can be defined as follows: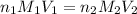
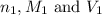 are the n-factor, molarity and volume of acidic solution while
are the n-factor, molarity and volume of acidic solution while 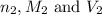 are the n-factor, molarity and volume of basic solution.
are the n-factor, molarity and volume of basic solution.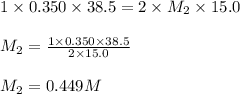
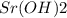 solution is 0.449 M.
solution is 0.449 M.


