

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:10
How have the greenhouse gasses increased from the year 2000 to 2018
Answers: 2

Chemistry, 22.06.2019 17:50
Cryolite, na3alf6(s), an ore used in the production of aluminum, can be synthesized using aluminum oxide. start this question by first balance the chemical equation.1.) balance the equation: - alo3(s)+naoh(l)+hf(> na3alf6+h2o(g). 2.) if 17.5 kilograms of al2o3(s), 51.4 kilograms of naoh(l), and 51.4 kilograms of hf(g) react completely, how many kilograms of cryolite will be produced? 3.)which reactants will be in excess, (al2o3, naoh, or hf) 4.)what is the total mass of the excess reactants left over after the reaction is complete in kg?
Answers: 2

Chemistry, 22.06.2019 18:00
Which three statements represent the benefits of performing experiments using computer simulations?
Answers: 2

You know the right answer?
How many moles of Cl2 are needed in order to produce 100.0 grams of FeCl3 given the following
equat...
Questions



Health, 14.11.2020 22:30


Chemistry, 14.11.2020 22:30

Mathematics, 14.11.2020 22:30

Mathematics, 14.11.2020 22:30


Mathematics, 14.11.2020 22:30


Mathematics, 14.11.2020 22:30






Biology, 14.11.2020 22:40

History, 14.11.2020 22:40

English, 14.11.2020 22:40

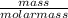
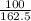 = 0.62mole
= 0.62mole 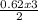 = 0.92mole
= 0.92mole 


