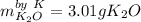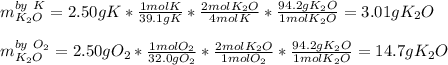Chemistry, 13.11.2020 06:40 EBeast7390
Given this reaction: 4K(s) + O2(g)→2K2O(s)
Calculate how many grams of product is produced if 2.50 g of each reactant is reacted.
PLEASE HURRY!!!

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1

Chemistry, 22.06.2019 12:00
An atom's configuration based on its number of electrons ends at 3p4. another atom has seven more electrons. starting at 3p, what is the remaining configuration? 3p63d34s2 3p43d54s2 3p64s23d3 3p44s23d
Answers: 3

Chemistry, 22.06.2019 12:30
According to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? view available hint(s) according to the valence shell electron pair repulsion (vsepr) theory, a molecule that has four electron groups around the central atom will exhibit what electron geometry? trigonal bipyramidal tetrahedral square planar determination of electron geometry requires information on whether the electron groups are lone pairs or bonding groups.
Answers: 2

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3
You know the right answer?
Given this reaction: 4K(s) + O2(g)→2K2O(s)
Calculate how many grams of product is produced if 2.50...
Questions




Social Studies, 28.09.2019 00:30

Mathematics, 28.09.2019 00:30






History, 28.09.2019 00:30


English, 28.09.2019 00:30

Physics, 28.09.2019 00:30

History, 28.09.2019 00:30


Mathematics, 28.09.2019 00:30

Chemistry, 28.09.2019 00:30


Mathematics, 28.09.2019 00:30