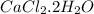Chemistry, 29.08.2019 07:00 mimireds5419
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within the desiccators and forms a hydrated salt. a 16.43g mass of anhydrous cacl2 weighed 21.75g after being left in desiccators for several months. what is the formula of the hydrated cacl2?

Answers: 1


Another question on Chemistry


Chemistry, 22.06.2019 07:00
The blackbody curve for a star name zeta is shown below. what is the peak wavelength for this star ?
Answers: 1


Chemistry, 22.06.2019 18:30
Which of the following words describe the reality that the universe looks the same from various perspective
Answers: 3
You know the right answer?
Anhydrous cacl2 is used as a desiccant in desiccators, i. e. it removes water from the air within th...
Questions

Computers and Technology, 10.03.2020 04:05








Mathematics, 10.03.2020 04:05




Computers and Technology, 10.03.2020 04:05

English, 10.03.2020 04:05