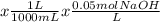Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 04:00
How do scientists think that gravity affected the formation of our solar system?
Answers: 1

Chemistry, 23.06.2019 08:30
Imagine you are a business executive who wants to pursue an environment policy for your company that limits pollution and uses fewer raw materials but would cost more what might be the discussion to your next broad meeting how would you make your case to your shareholders
Answers: 1

Chemistry, 23.06.2019 11:40
An electron moved from a lower energy level to a higher energy level. what most likely happened during the transition? a random amount of light was released. a fixed amount of energy was absorbed. a fixed amount of energy was released. a random amount of light was absorbed.
Answers: 1

You know the right answer?
In a titration, you start with 0.05 M sodium hydroxide (NaOH) and slowly add it to 40 mL of HC1 with...
Questions

Mathematics, 19.12.2019 08:31


Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31


Mathematics, 19.12.2019 08:31

Physics, 19.12.2019 08:31

Physics, 19.12.2019 08:31


History, 19.12.2019 08:31

Health, 19.12.2019 08:31

Biology, 19.12.2019 08:31

Social Studies, 19.12.2019 08:31

Biology, 19.12.2019 08:31

History, 19.12.2019 08:31


English, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31

Mathematics, 19.12.2019 08:31