Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
There are 6.022, 104 atoms of hg in 1 mole of hg the number of atoms in 45 moles of hg can be found by multiplying 4.5 by 6.022, 102 which is the number of atoms in 4.5 moles of hg, correctly written in scientific notation with the correct number of significant figures? 0 21,109 0 21,100 271, 1024 27.099, 100 mark this and retum save and exit submit
Answers: 1


Chemistry, 22.06.2019 23:30
Rank substituents in order of their priority when assigning the e or z label to an alkene. i, ch2i , h, ch2ch2cl, f
Answers: 2

Chemistry, 23.06.2019 00:00
If many scientists conduct the same or similar experiments, and all obtain similar results, a can be written, which is a generally agreed-upon statement that explains and predicts how a natural phenomenon works.
Answers: 1
You know the right answer?
Write the balanced chemical equation for the following reaction. Phases are optional. Solid iron(I)...
Questions




Physics, 15.02.2020 03:07

Mathematics, 15.02.2020 03:07




Mathematics, 15.02.2020 03:09

Mathematics, 15.02.2020 03:09






Computers and Technology, 15.02.2020 03:13




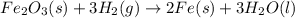
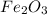 , hydrogen gas is
, hydrogen gas is 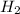 , solid iron is Fe and liquid water is just
, solid iron is Fe and liquid water is just 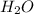 , we can write:
, we can write: