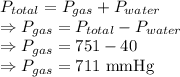Chemistry, 11.11.2020 09:00 Irishstoner5608
A laboratory hydrogen generator collects the gas produced by bubbling it through water. The total pressure of the gas collected is 751 mmHg. The temperature is 34 °C, at which water vapor pressure is 40.0 mmHg. Calculate the partial pressure of the hydrogen.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An atom of lithium (li) and an atom of chlorine (cl) engage in a chemical reaction. which correctly describes the structure of the resulting chemical compound? hint: consider the class of each element. the chemical compound will have a network structure. the chemical compound will have triple bonds. the chemical compound will have a ball-and-stick structure. the chemical compound will have double bonds.
Answers: 2

Chemistry, 22.06.2019 17:00
How can a give a full method for the experiment of separating sand from water by filtration? 1-materials 2-steps 3-conclusion also for water and salt separated by the evaporation or distillation process
Answers: 1

Chemistry, 22.06.2019 18:30
Which of the following nuclei would be the least stable a 2 protons, 2 neutrons b 1 proton 1 neutron c 1 proton 3 neutrons d 1 proton 2 neutrons
Answers: 3

Chemistry, 23.06.2019 00:30
What is bromine+calcium iodide--> calcium bromide +iodine balanced
Answers: 1
You know the right answer?
A laboratory hydrogen generator collects the gas produced by bubbling it through water. The total pr...
Questions

Mathematics, 03.09.2020 04:01

Biology, 03.09.2020 04:01



Social Studies, 03.09.2020 04:01




Mathematics, 03.09.2020 04:01


History, 03.09.2020 04:01



Mathematics, 03.09.2020 04:01





Geography, 03.09.2020 04:01

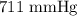
 = Total pressure = 751 mmHg
= Total pressure = 751 mmHg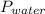 = Vapor pressure = 40 mmHg
= Vapor pressure = 40 mmHg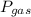 = Pressure of gas
= Pressure of gas