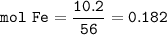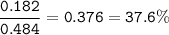Chemistry, 11.11.2020 06:10 connienash95
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a) Actual yield of Fe mole
(b) % mole
(c) theoretical yield of iron mmoles

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
According to each substances heat of fusion, which of the items below requires more heat to be added per gram of substance to go from solid to liquid? silver sulfur water lead
Answers: 2

Chemistry, 22.06.2019 06:30
Particle model to predict what will happen if a sharp object creates a hole in the soccer ball
Answers: 2

Chemistry, 22.06.2019 18:00
Chlorophyll a had the molecular formula c55h72mgn4o5 how many atoms are in this molecule
Answers: 2

Chemistry, 22.06.2019 19:00
Convert the temperature of dry ice, –77 ∞c, into degrees fahrenheit and kelvin.
Answers: 2
You know the right answer?
2. Combining 0.242 mol Fe2O3 with excess carbon produced 10.2 g Fe.
Fe2O3+3C⟶2Fe+3CO
(a)...
(a)...
Questions

History, 20.10.2021 01:00


Mathematics, 20.10.2021 01:00


Chemistry, 20.10.2021 01:00

Mathematics, 20.10.2021 01:00

Mathematics, 20.10.2021 01:00


Mathematics, 20.10.2021 01:00



Social Studies, 20.10.2021 01:00


Mathematics, 20.10.2021 01:00


History, 20.10.2021 01:00

Mathematics, 20.10.2021 01:00