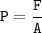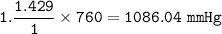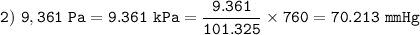Chemistry, 10.11.2020 20:10 crowzombie9
Perform each of the following unit conversions using the conversion factors given below:
1.000 atm = 760.0 mmHg = 101.325 kPa
1.429 atm =
mmHg
9,361 Pa =
kPa =
mmHg
725 mmHg =
atm =
kPa

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:50
Blank allows you to do calculations for situations in which only the amount of gas is constant a)boyle's law b)combined gas law c)ideal gas law d)dalton's law
Answers: 1

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 00:40
Which is a difference between molecular compounds and ionic compounds? select the correct answer below: question 5 options: molecular compounds typically form between a metal and a nonmetal, while ionic compounds typically form between nonmetals. molecular compounds result from the transfer of electrons between atoms to form ions, while ionic compounds result from the sharing of electrons between neutral atoms. molecular compounds are formed of discrete, neutral molecules, while ionic compounds are formed of large repeating arrays of opposite charges. molecular compounds have high melting points and high boiling points, while ionic
Answers: 3

Chemistry, 22.06.2019 19:00
How many moles of cu are needed to react with 5.8 moles of agno3? cu + 2 agno3 → cu(no3)2 + 2 ag
Answers: 3
You know the right answer?
Perform each of the following unit conversions using the conversion factors given below:
1.000 atm...
Questions


Mathematics, 25.06.2019 11:00

Mathematics, 25.06.2019 11:00

Mathematics, 25.06.2019 11:00

Mathematics, 25.06.2019 11:00


Health, 25.06.2019 11:00

Biology, 25.06.2019 11:00

Biology, 25.06.2019 11:00


Mathematics, 25.06.2019 11:00


Mathematics, 25.06.2019 11:00



Mathematics, 25.06.2019 11:00

History, 25.06.2019 11:00



Mathematics, 25.06.2019 11:00