
Chemistry, 10.11.2020 20:10 StephenSudu
A 50.0 ml sample of 0.200 M HNO2 (weak acid) is titrated with 0.400 M
KOH. Calculate the pH after addition of 25 ml KOH (Ky for HNO, is 4.6 x
10-4)
O a 6.67
O b. 7.67
O c.8.23
O d. 9.12

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:40
For each of the following compounds, write the formula then predict whether it would be a strong, weak, or non-electrolyte when placed in di water. for the ionic compounds only, put (s) or (aq) after the forrmula formula strong, weak or non electrolyte? a calcium hydroxide b. silver carbonate c. lead(ii) sulfate d. phosphorus trifluoride e. sodium phosphide f barium sulfate g. strontium acetate h. zinc nitrate
Answers: 3


Chemistry, 22.06.2019 13:00
Which of the following are good traits of a hypothesis? it will be able to be testedit can predict an outcomeit will explain the observationsall of these
Answers: 2

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2
You know the right answer?
A 50.0 ml sample of 0.200 M HNO2 (weak acid) is titrated with 0.400 M
KOH. Calculate the pH after a...
Questions


Spanish, 19.03.2021 02:20



Mathematics, 19.03.2021 02:20





Business, 19.03.2021 02:20





Mathematics, 19.03.2021 02:20

Mathematics, 19.03.2021 02:20

Mathematics, 19.03.2021 02:20



![\tt [OH^-]=\sqrt{\dfrac{Kw}{Ka}.M }](/tpl/images/0884/8776/41931.png)
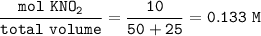
![\tt [OH^-]=\sqrt{\dfrac{10^{14}}{4.6\times 10^{-4}} }\times 0.133\\\\(OH^-)=1.7\times 10^{-6}\\\\pOH=6-log~1.7=5.77\\\\pH+pOH=14\\\\pH+5.77=14\\\\pH=8.23](/tpl/images/0884/8776/d039c.png)


