Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:10
What does a particular point on a line of a phase diagram represent? o a. the maximum temperature a substance can exist at without bonds breaking b. the pressure created by the kinetic energy of molecules at a particular temperature c. the melting point or boiling point of a substance at a specific pressure d. the conditions in which temperature and pressure have equal effects on a substance
Answers: 2

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

Chemistry, 22.06.2019 22:30
Essay-alternative energy sources research sources of energy that are being developed. write a report of 350-400 words discussing the information you learned concerning the development of various energy sources and the impact that you think they will have on your life. include sources cited at the end of your report using the mla format. follow the rubric guidelines. note that wikipedia is not an appropriate resource for a research paper. worth 99
Answers: 3

You know the right answer?
PLEASE HELP
Write the full electronic configuration and draw the orbital box diagram of iron in its...
Questions

Mathematics, 16.04.2022 18:10


English, 16.04.2022 18:30


English, 16.04.2022 18:50



Mathematics, 16.04.2022 19:20


English, 16.04.2022 20:20



Mathematics, 16.04.2022 20:30



Business, 16.04.2022 21:10




 :1
:1
 2
2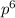 3
3