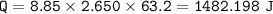Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:00
8.98 dm3 of hydrogen gas is collected at 38.8 °c. find the volume the gas will occupy at -39.9 °c if the pressure remains constant.
Answers: 3

Chemistry, 22.06.2019 14:00
Ascientist measures the speed of sound in a monatomic gas to be 449 m/s at 20∘c. what is the molar mass of this gas?
Answers: 2

Chemistry, 22.06.2019 15:10
Which statement describes the phase change that occurs when dry ice is placed in an open container at room temperature?
Answers: 1
You know the right answer?
The internal energy of reaction is -855.1). The reaction has a change of
temperature of 63.20°C tha...
Questions



Chemistry, 17.11.2020 15:00

English, 17.11.2020 15:00






English, 17.11.2020 15:10

Mathematics, 17.11.2020 15:10



Mathematics, 17.11.2020 15:10



Physics, 17.11.2020 15:10


Mathematics, 17.11.2020 15:10