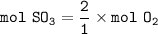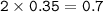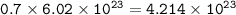Chemistry, 06.11.2020 23:30 claytonashley30
How many molecules of SO₃ can be formed from 0.35 moles of O₂ (assuming excess SO₂) from the following UNBALANCED equation? SO₂(g) + O₂(g) → SO₃(g)

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 17:30
Aroller coaster is traveling at 13 mi./s when you purchase a hill that is 400 m long and down the hill exonerate at 4.0 m/s squared what is the final velocity of the posterior found your answer to the nearest number
Answers: 1


Chemistry, 23.06.2019 11:00
Asolubility table shows that almost all compounds of group 1 metals are soluble in water. this general rule tells you that mgi2 is soluble rbno3 is soluble cacl2 is soluble co2 is soluble
Answers: 1

Chemistry, 23.06.2019 16:00
Henry moseley used x-ray experiments to determine the atomic number of elements. how did his discovery contribute to the development of the periodic table? a.it confirmed that elements should be arranged in strict order of increasing atomic mass. b.it led to elements with similar atomic numbers being grouped together. c.it allowed the elements to be placed in strict order of increasing atomic number. d.it showed that the way mendeleev grouped elements together was completely wrong.
Answers: 1
You know the right answer?
How many molecules of SO₃ can be formed from 0.35 moles of O₂ (assuming excess SO₂) from the followi...
Questions

Biology, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00

Business, 09.02.2021 22:00


Arts, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00


Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00


Chemistry, 09.02.2021 22:00





Mathematics, 09.02.2021 22:00

Mathematics, 09.02.2021 22:00