Chemistry, 06.11.2020 22:50 davidswafforddd478
Boyles Law P1V1 = P2V2
An arrangement of helium balloons in the back of a delivery truck travels from a town at the base of a mountain up the mountain where the air pressure is half the amount. What do you expect to happen to the balloons?
A. The balloons will increase to twice their original volume.
B. The balloons will decease to one fourth their original size.
.C. The balloons will increase to four times their original size.
D. The balloons will decrease to half their original size.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Which statements are true about electrolysis? check all that apply. electrolysis requires an acid be present. electrolysis is described by two half-reactions. electrolysis is not an industrial process. electrolysis results in commercially valuable products. electrolysis involves the transfer of electrons. reduction results in the loss of electrons. oxidation results in the loss of electrons.
Answers: 1

Chemistry, 21.06.2019 22:00
Which of the following statements is true about planck’s law
Answers: 1

Chemistry, 22.06.2019 04:50
Compare the equilibrium constants for the systems shown in the table. which favors products the most? which favors products the least? rank these systems in order from most to least in terms of favoring products rather than reactants. d > b > a > c c > a > b > d b > c > d > a a > d > c > b
Answers: 1

Chemistry, 22.06.2019 10:10
Stage in which a typical star has completely stopped fusion
Answers: 1
You know the right answer?
Boyles Law P1V1 = P2V2
An arrangement of helium balloons in the back of a delivery truck travels fr...
Questions


Mathematics, 18.09.2021 21:50


Mathematics, 18.09.2021 21:50




Chemistry, 18.09.2021 21:50

Business, 18.09.2021 21:50



Mathematics, 18.09.2021 21:50

English, 18.09.2021 21:50


Physics, 18.09.2021 21:50

History, 18.09.2021 21:50


English, 18.09.2021 21:50

Computers and Technology, 18.09.2021 21:50

Mathematics, 18.09.2021 21:50

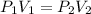
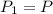 , initial volume be V, i.e.
, initial volume be V, i.e. 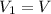 . The pressure is then halved, i.e.
. The pressure is then halved, i.e.