
Chemistry, 06.11.2020 17:10 delanieloya
Suppose that 0.410 mol of methane, CH4(g), is reacted with 0.560 mol of fluorine, F2(g), forming CF4(g) and HF(g) as sole products. Assuming that the reaction occurs at constant pressure, how much heat is released

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 23:00
A100-watt light bulb radiates energy at a rate of 100 j/s. (the watt, a unit of power or energy over time, is defined as 1 j/s.) if all of the light emitted has a wavelength of 525 nm , how many photons are emitted per second?
Answers: 1

Chemistry, 22.06.2019 00:00
For ai it's atomic number is 13 and it's mass number is 27 how many neutrons does it have
Answers: 1

Chemistry, 22.06.2019 20:10
Suppose you mix one mole of sulfuric acid (h2so4) with 1 mole of sodium hydroxide(naoh). why does the ph of the solution remain below 7? ( explain so i can get better understanding! )
Answers: 2

Chemistry, 23.06.2019 01:30
The biomedical technique in which a part of the brain is destroyed with electric current is known as a. electroconvulsive therapy b. prefrontal lobotomy c. bilateral cingulotomy d. tardive dyskinesia
Answers: 2
You know the right answer?
Suppose that 0.410 mol of methane, CH4(g), is reacted with 0.560 mol of fluorine, F2(g), forming CF4...
Questions

Mathematics, 19.04.2020 13:54

Mathematics, 19.04.2020 13:54

Mathematics, 19.04.2020 13:54


Health, 19.04.2020 13:54

Mathematics, 19.04.2020 13:54


Mathematics, 19.04.2020 13:55

Mathematics, 19.04.2020 13:56

Mathematics, 19.04.2020 13:56

Mathematics, 19.04.2020 13:57


Mathematics, 19.04.2020 13:57

Biology, 19.04.2020 13:59

Mathematics, 19.04.2020 13:59





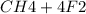 →
→ 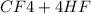
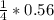 = 0.14mole of CF44 moles of fluorine → 4 moles of HF0.56 mole of fluorine → 0.56 mole of HF
= 0.14mole of CF44 moles of fluorine → 4 moles of HF0.56 mole of fluorine → 0.56 mole of HF


