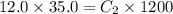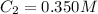Chemistry, 06.11.2020 16:40 yclark98563p5z2gt
What is the final concentration of a solution prepared by diluting 35.0 mL of 12.0 M HCl to a final volume of 1.20 L

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:30
Used the balanced equation 2h2+ o2 - -> 2h2o. if you have 7.2 grams of o2 , how many grams of h2o can you produce ?
Answers: 2

Chemistry, 22.06.2019 13:00
Using the thermodynamic information in the aleks data tab, calculate the standard reaction free energy of the following chemical reaction: →+p4o10s6h2ol4h3po4s round your answer to zero decimal places.
Answers: 3

Chemistry, 22.06.2019 13:00
The number of neutrons is equal to the atomic number minus the atomic mass. a. true b. false
Answers: 2

Chemistry, 22.06.2019 13:50
What happens when an atom of sulfur combines with two atoms of chlorine to produce sci2? a. each chlorine atom shares a pair of electrons with the sulfur atom. b. an electron is transferred from each chlorine atom to the sulfur atom. c. an electron is transferred from the sulfur atom to each chlorine atom. d. each chlorine atom shares all its valence electrons with the sulfur atom.
Answers: 2
You know the right answer?
What is the final concentration of a solution prepared by diluting 35.0 mL of 12.0 M HCl to a final...
Questions




Health, 28.09.2019 11:30


History, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30


Spanish, 28.09.2019 11:30



History, 28.09.2019 11:30


Mathematics, 28.09.2019 11:30


Mathematics, 28.09.2019 11:30

Chemistry, 28.09.2019 11:30

Mathematics, 28.09.2019 11:30

Social Studies, 28.09.2019 11:30

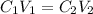
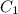 = concentration of concentrated acid solution = 12.0 M
= concentration of concentrated acid solution = 12.0 M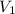 = volume of concentrated acid solution = 35.0 ml
= volume of concentrated acid solution = 35.0 ml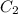 = concentration of diluted acid solution= ?
= concentration of diluted acid solution= ?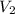 = volume of another acid solution= 1.20 L = 1200 ml
= volume of another acid solution= 1.20 L = 1200 ml