Chemistry, 06.11.2020 16:40 ahnaodoido384
An excess of oxygen reacts with 451.4 g of lead, forming 374.7 g of lead(II) oxide. Calculate the percent yield of the reaction.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Atrain travels 74 kilometers in 3 hours, and then 81 kilometers in 5 hours. what is its average speed?
Answers: 2

Chemistry, 22.06.2019 16:30
At 20°c, a sample of h2o liquid and a sample of co2 gas each have the same average kinetic energy. why is one a liquid and the other a gas at this temperature?
Answers: 1

Chemistry, 23.06.2019 09:30
What is the best describtion of the side of the moon that faces earth?
Answers: 2

Chemistry, 23.06.2019 13:30
Consider this reaction taking place in a closed 2 liter container: 2so2(g) + o2(g) → 2so3(g) if the volume of the container is decreased to 1 liter, what will happen to the equilibrium of the reaction? it will shift left. it will shift right. it will remain constant it will decrease by half
Answers: 3
You know the right answer?
An excess of oxygen reacts with 451.4 g of lead, forming 374.7 g of lead(II) oxide. Calculate the pe...
Questions








English, 12.12.2019 21:31

Spanish, 12.12.2019 21:31


English, 12.12.2019 21:31


History, 12.12.2019 21:31

English, 12.12.2019 21:31

Physics, 12.12.2019 21:31



English, 12.12.2019 21:31


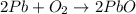
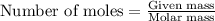
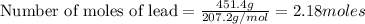
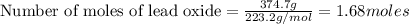
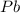 produces = 2 moles of
produces = 2 moles of 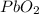
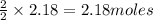 of
of