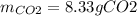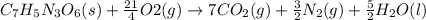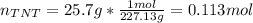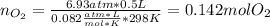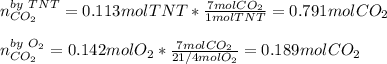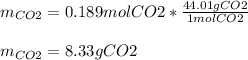Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced chemical equation:
C7H5N3O6(s)+214O2(g)→7CO2(g)+32N2(g )+52H2O(l)
If 25.7 g of TNT is combusted in a 0.500 L container filled with O2 at a pressure of 7.02 bar and a temperature of 298 K, calculate the maximum mass of CO2 that could be produced.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
Write a brief passage describing a neutral atom of nitrogen-14 (n-14). describe the number of protons, neutrons, and electrons in the atom, where each type of particle is located, and how the terms atomic number, mass number, and atomic mass are related to the particles. use the periodic table to you. 14 protons and eletrons since its a neutral atom
Answers: 1

Chemistry, 21.06.2019 23:30
Start an single atom tab. observe the decay of polonium-211. after each decay, press the reset nucleus button to watch the process again. write a description of alpha decay for po-211
Answers: 2

Chemistry, 22.06.2019 23:10
Match the formula for the following compound: magnesium sulfate heptahydratemgs·7h2omg2so4·7h2omg(so4)2·7h2omgso4·7h2o
Answers: 1

Chemistry, 23.06.2019 01:00
Which process results in the release of energy stored in the products of photosynthesis? a. polymer synthesis b. depolymerization c. digestion d. cellular respiration
Answers: 1
You know the right answer?
Trinitrotoluene (TNT, C7H5N3O6) undergoes complete combustion according to the following balanced ch...
Questions

English, 26.08.2019 00:10

Physics, 26.08.2019 00:10





English, 26.08.2019 00:10

Mathematics, 26.08.2019 00:10

Chemistry, 26.08.2019 00:10

Social Studies, 26.08.2019 00:10


History, 26.08.2019 00:10






Mathematics, 26.08.2019 00:10