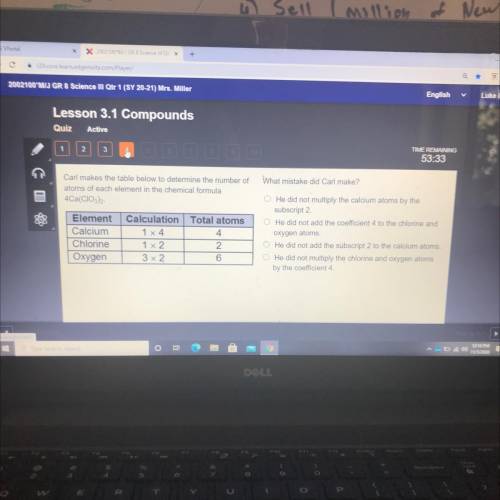
Whet mistake did Cad mekel
Carl makes the table below to determine the number of
atoms of eac...

Whet mistake did Cad mekel
Carl makes the table below to determine the number of
atoms of each element in the chemical formula
4Ca(CIO)
Element
Calcium
Chlorine
Oxygen
Calculation Total atoms
1x4
4
172
2
372
He did not multiply the calcium stoms by the
subscript 2
He did not add the coefficient to the choirea
orygen atoms
He did not add the subscriot 2 to tre calor ao
He did not multiply the chone and one or
by the coeficient 4

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 00:00
The p sub shell can hold up to 8 electrons in an atom. true or false?
Answers: 1

Chemistry, 22.06.2019 12:20
Achemistry student weighs out 0.306 g of citric acid (h3c6h5o7), a triprotic acid, into a 250 ml volumetric flask and dilutes to the mark with distilled water. he plans to titrate the acid with 0.1000 m naoh solution. calculate the volume of naoh solution the student will need to add to reach the final equivalence point. be sure your answer has the correct number of significant digits.
Answers: 3

Chemistry, 23.06.2019 09:30
My plate recommends that of your nutritional intake comes from fruits and vegetables. a. 30% b. 50% c. 20% d. 40%
Answers: 2

Chemistry, 23.06.2019 16:30
Match the words in the left column to the appropriate blanks in the sentences on the right. (a) this is the law of definite proportions: all samples of a given compound, regardless of their source or how they were prepared, have proportions of their constituent elements. (b) this is the law of conservation of mass: in a reaction, matter is neither created nor destroyed. (c) this is the law of multiple (c) this is the law of multiple blank: when two elements form two different compounds, the masses of element b that combine with 1 g of element a can be expressed as a ratio of small whole-numbers. in this example, the ratio of \rm o from hydrogen peroxide to \rm o from water = 16: 8 \to 2: 1, a small whole-number ratio.: when two elements form two different compounds, the masses of element b that combine with 1 g of element a can be expressed as a ratio of small whole-numbers. in this example, the ratio of o from hydrogen peroxide to o from water = 16: 8 → 2: 1, a small whole-number ratio.
Answers: 1
You know the right answer?
Questions

History, 26.10.2019 12:43


English, 26.10.2019 12:43


Biology, 26.10.2019 12:43

Social Studies, 26.10.2019 12:43


English, 26.10.2019 12:43

Geography, 26.10.2019 12:43


Mathematics, 26.10.2019 12:43


Biology, 26.10.2019 12:43

History, 26.10.2019 13:43

Mathematics, 26.10.2019 13:43


English, 26.10.2019 13:43





