
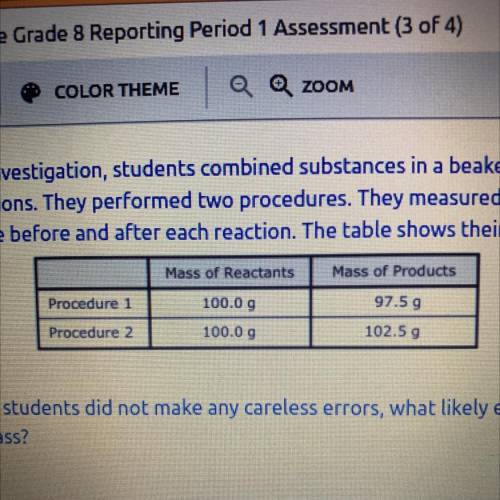
As part of an investigation, students combined substances in a beaker to observe
chemical reactions. They performed two procedures. They measured the mass of
each substance before and after each reaction. The table shows their observations.
Mass of Products
Procedure
97.59
Procedure 2
102.50
Procedure 1: All the reactants were liquids that evaporated.
Procedure 2: A gas was formed as one product, and it escaped into
the air
Mass of Reactants
100.0 9
100.00
Procedure 1: One of the reactants was converted to thermal
energy,
Procedure 2: All the products were liquids.
e
Assuming the students did not make any careless errors, what likely explains these
changes in mass?
Procedure 1: The reactants were liquids with different densities.
Procedure 2: The reactants were combined into only one product.
e
air.
Procedure 1: One of the products was a gas that escaped into the
Procedure 2: A gas from the air reacted with one of the other
reactants and formed a precipitate.
CLEAR ALL

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 02:30
When you perform this reaction, what could remain at the end of the reaction? check all that apply. excess reactant aqueous copper chloride excess reactant aluminum oxygen product solid copper carbon dioxide product aqueous aluminum chloride water
Answers: 2

Chemistry, 22.06.2019 04:50
Write the overall equation for the reaction for lithium battery
Answers: 2

Chemistry, 22.06.2019 14:40
Choose an equation that represents an enzyme-catalyzed reaction. (a) enzyme + substrate → enzyme-substrate complex (b) enzyme + substrate ←−→ enzyme + products (c) enzyme + substrate ←−→ enzyme-substrate complex → enzyme + products (d) enzyme + substrate ←−→ enzyme-substrate complex → enzyme-substrate complex + products
Answers: 2

Chemistry, 22.06.2019 17:00
According to the kinetic-molecular theory, what happens to a liquid when it is transferred from one container to another? the volume and the shape stay the same. the volume increases to fill the new container, but the shape stays the same. the volume stays the same, but the shape changes to fit the new container. the volume and the shape change to fill the new container.
Answers: 2
You know the right answer?
As part of an investigation, students combined substances in a beaker to observe
chemical reactions...
Questions

Business, 08.07.2019 16:30

Biology, 08.07.2019 16:30

Biology, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30

Social Studies, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30

Biology, 08.07.2019 16:30


Chemistry, 08.07.2019 16:30

Mathematics, 08.07.2019 16:30


Mathematics, 08.07.2019 16:30


Geography, 08.07.2019 16:30

Spanish, 08.07.2019 16:30



English, 08.07.2019 16:30


Mathematics, 08.07.2019 16:30



