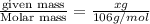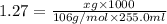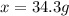Chemistry, 05.11.2020 18:30 jjjoooorrrrddddaannn
A chemist adds 255.0mL of a 1.27M sodium carbonate Na2CO3 solution to a reaction flask. Calculate the mass in grams of sodium carbonate the chemist has added to the flask.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:10
There are 6.022 x 10^23 atoms of hg in 1 mole of hg. the number of atoms in 4.5 moles of hg can be found by multiplying 4.5 by 6.022 x 10^23 a. 2.7 x 10^24 b. 27 x 10^23 c. 2.71 x10^24 d. 27.099 x 10^23
Answers: 3

Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1


Chemistry, 22.06.2019 12:30
In france, grapes are 1.95 euros per kilogram. what is the cost of grapes, in dollars per pound, if the exchange rate is 1.14 dollars/euro? (2.6)
Answers: 3
You know the right answer?
A chemist adds 255.0mL of a 1.27M sodium carbonate Na2CO3 solution to a reaction flask. Calculate th...
Questions



Mathematics, 06.07.2019 10:30

Biology, 06.07.2019 10:30

Biology, 06.07.2019 10:30

Mathematics, 06.07.2019 10:30


Social Studies, 06.07.2019 10:30


Biology, 06.07.2019 10:30


Mathematics, 06.07.2019 10:30

Mathematics, 06.07.2019 10:30

Health, 06.07.2019 10:30



History, 06.07.2019 10:30



History, 06.07.2019 10:30

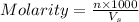
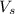 = volume of solution in ml
= volume of solution in ml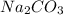 =
=