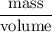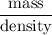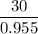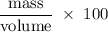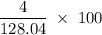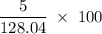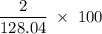Chemistry, 05.11.2020 16:40 lauramount
Flexible collodion contains 20% w/w camphor and 30% w/w castor oil. how many grams of each would be contained in 30 g of the mixture? (b) the specific gravity of castor oil is 0.955. how many milliliters of the oil is contained in 30 g of the mixture? (c) if the specific gravity of the mixture is 0.781, what are the percent w/v concentrations of lactic acid, salicylic acid, and trichloroacetic acid in the mixture?

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2

Chemistry, 22.06.2019 10:30
Aglow stick contains a glass vial with chemicals. when the glow stick is bent, the vial breaks and the chemicals react to produce a glow. a science student observes that a glow stick kept in the freezer glows for a longer duration than a glow stick kept at room temperature. what conclusion can be drawn based on the observation? be sure to note the outcome and test variables in the conclusion.
Answers: 1

Chemistry, 22.06.2019 10:50
Someone offer some answers to this, i will give 98 coins and mark as brainliest! i will put the rest of the lab down in the comments,solutions pre-lab questions: in this lab, you will make fruit drinks with powdered drink mix. complete the pre-lab questions to get the values you need for your drink solutions. calculate the molar mass of powered fruit drink mix, made from sucrose (c12h22o11).using stoichiometry, determine the mass of powdered drink mix needed to make a 1.0 m solution of 100 ml. (hint: use molarity = to find the moles of drink mix, then convert moles to grams using a mole conversion.)what mass of powdered drink mix is needed to make a 0.5 m solution of 100 ml?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Flexible collodion contains 20% w/w camphor and 30% w/w castor oil. how many grams of each would be...
Questions


English, 22.10.2019 13:50

Biology, 22.10.2019 13:50


Biology, 22.10.2019 13:50

Physics, 22.10.2019 13:50

Physics, 22.10.2019 13:50

Mathematics, 22.10.2019 13:50

Mathematics, 22.10.2019 13:50

Physics, 22.10.2019 13:50

Mathematics, 22.10.2019 14:00

Mathematics, 22.10.2019 14:00

Chemistry, 22.10.2019 14:00


Mathematics, 22.10.2019 14:00

Mathematics, 22.10.2019 14:00

History, 22.10.2019 14:00


History, 22.10.2019 14:00

Biology, 22.10.2019 14:00