
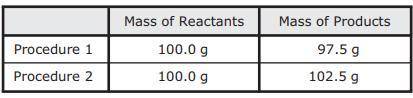
As part of an investigation, students combined substances in a beaker to observe chemical reactions. They performed two procedures. They measured the mass of each substance before and after each reaction. The table shows their observations.
Assuming the students did not make any careless errors, what likely explains these changes in mass?
A. Procedure 1: All the reactants were liquids that evaporated.
Procedure 2: A gas was formed as one product, and it escaped into the air.
B. Procedure 1: One of the reactants was converted to thermal energy.
Procedure 2: All the products were liquids.
C. Procedure 1: The reactants were liquids with different densities.
Procedure 2: The reactants were combined into only one product.
D. Procedure 1: One of the products was a gas that escaped into the air.
Procedure 2: A gas from the air reacted with one of the other reactants.

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Which type of bond is present in hydrogen sulfide (h2s)? the table of electronegativities is given. a. hydrogen b. ionic c. nonpolar covalent d. polar covalent
Answers: 1

Chemistry, 22.06.2019 11:00
What is the molar mass of a gas that has density of 2.054 g/l
Answers: 2

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

You know the right answer?
As part of an investigation, students combined substances in a beaker to observe chemical reactions....
Questions



History, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00

History, 09.12.2020 14:00

Business, 09.12.2020 14:00


Biology, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00

Mathematics, 09.12.2020 14:00


Mathematics, 09.12.2020 14:00


Advanced Placement (AP), 09.12.2020 14:00

Chemistry, 09.12.2020 14:00

History, 09.12.2020 14:00



