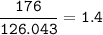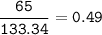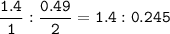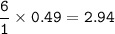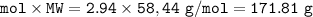Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 17:30
Aaspirin has a density of 1.40 g/cm^3 what is the volume in cubic centimeters of a tablet weighing 320 mg?
Answers: 3


Chemistry, 22.06.2019 13:30
Table sugar completely dissolved in water is an example of a?
Answers: 1

Chemistry, 22.06.2019 13:50
Abeaker with 2.00×102 ml of an acetic acid buffer with a ph of 5.000 is sitting on a benchtop. the total molarity of acid and conjugate base in this buffer is 0.100 m. a student adds 4.70 ml of a 0.360 m hcl solution to the beaker. how much will the ph change? the pka of acetic acid is 4.740.
Answers: 1
You know the right answer?
Na2SO3 + 2 AlCl3 --> Al2(SO3)3 + 6 NaCl Using the above reaction, suppose (1.760x10^2) grams of N...
Questions







Mathematics, 11.01.2020 02:31


Mathematics, 11.01.2020 02:31

History, 11.01.2020 02:31





History, 11.01.2020 02:31


Health, 11.01.2020 02:31



English, 11.01.2020 02:31