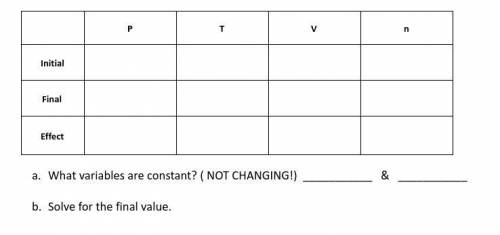
A gas occupies a volume of 180 mL at 35oC and 740 mmHg. What is the volume of the gas at STP?
...

Chemistry, 05.11.2020 03:30 korbenbrown7554
A gas occupies a volume of 180 mL at 35oC and 740 mmHg. What is the volume of the gas at STP?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Under normal conditions, describe how increasing the temperatures effects the solubility of a typical salt
Answers: 1

Chemistry, 22.06.2019 14:30
Amixture that has two or more substances that are spread out evenly is called a. compound b. heterogeneous c. substance d. homogeneous
Answers: 1

Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 05:30
Elizabeth has two separate samples of the same substance. sample is in the liquid state, and the other is in the solid state. the two samples most likely differ in which property?
Answers: 1
You know the right answer?
Questions

Physics, 30.08.2020 01:01




Computers and Technology, 30.08.2020 01:01

History, 30.08.2020 01:01

Chemistry, 30.08.2020 01:01


History, 30.08.2020 01:01



Mathematics, 30.08.2020 01:01

Geography, 30.08.2020 01:01




Arts, 30.08.2020 01:01

Mathematics, 30.08.2020 01:01


Mathematics, 30.08.2020 01:01



