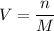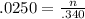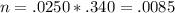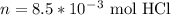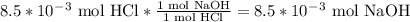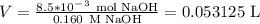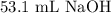Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 21:00
Use the measurements in the table to determine which unidentified metal has the highest density. metal volume mass a 10.5 cm3 122 g b 14.2 cm3 132 g c 16.1 cm3 115 g d 12.7 cm3 126 g
Answers: 2

Chemistry, 23.06.2019 00:20
4. propanol and isopropanol are isomers. this means that they have a) the same molecular formula but different chemical properties. b) different molecular formulas but the same chemical properties. c) the same molecular formula and the same chemical properties. d) the same molecular formula but represent different states of the compound
Answers: 3

You know the right answer?
Consider the titration of 25.0 mL of 0.340 M HCl with 0.160 M NaOH. What volume of NaOH is required...
Questions

Mathematics, 25.01.2021 04:50

Mathematics, 25.01.2021 04:50



Mathematics, 25.01.2021 04:50

Mathematics, 25.01.2021 04:50


Arts, 25.01.2021 04:50


Mathematics, 25.01.2021 04:50



Mathematics, 25.01.2021 04:50

Chemistry, 25.01.2021 04:50

Mathematics, 25.01.2021 04:50

History, 25.01.2021 04:50

Chemistry, 25.01.2021 04:50

Chemistry, 25.01.2021 04:50


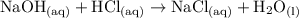
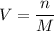 where V = volume (L)n = number of moles M = molarity
where V = volume (L)n = number of moles M = molarity