
Chemistry, 04.11.2020 19:50 haleybug100300
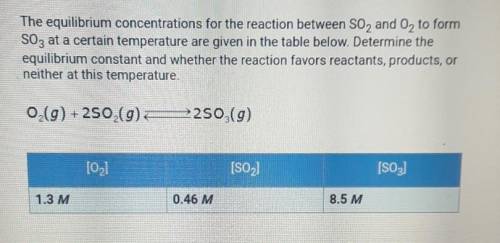
the equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperature are given in the table below. determine the equilibrium constant and whether the reaction favors reactants, products, or neither at this temperature.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:00
Read these sentences from the text. near the equator, the tropics receive the most rain on a consistent basis. as a result, the fresh water falling into the ocean decrease the salinity of the surface water in that region. [. .] . . as the salt content of sea water increases, so does its density. what can you infer about how rain affects the density of surface water near the equator?
Answers: 1

Chemistry, 22.06.2019 05:30
Modern weaponry has increased the number of deaths in wars and violent conflicts.
Answers: 3

Chemistry, 22.06.2019 10:00
The reactions shown here can be combined to make the overall reaction c(s) + h2o(g) ⇌ co(g) + h2(g) by reversing some and/or dividing all the coefficients by a number. a. c(s) + o2(g) → co2(g) k=1.363×10^69 b. 2 h2(g) + o2(g) → 2 h2o(g) k=1.389×10^80 c. 2co(g) + o2 (g) → 2 co2(g) k=1.477×10^90
Answers: 1

Chemistry, 22.06.2019 14:30
In water, a strong acid will break down into its component parts. a. completely b. partly c. never in water, a weak base will break down into its component parts. a. completely b. partly c. never
Answers: 2
You know the right answer?
the equilibrium concentrations for the reaction between SO2 and O2 to form SO3 at a certain temperat...
Questions

Mathematics, 30.03.2021 03:40



Computers and Technology, 30.03.2021 03:40


Mathematics, 30.03.2021 03:40



Mathematics, 30.03.2021 03:40


Mathematics, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40

History, 30.03.2021 03:40

Biology, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40

Mathematics, 30.03.2021 03:40

Advanced Placement (AP), 30.03.2021 03:40



