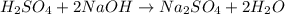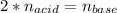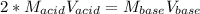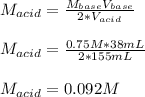Chemistry, 04.11.2020 19:00 joylsbarbour
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H2SO4). a. Write a balanced equation for the neutralizationof NaOH with H2SO4

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 22:30
For the following, determine the type of reaction and then give products.
Answers: 2

Chemistry, 22.06.2019 00:30
Jessica is traveling from miami, florida, to chicago, illinois. using the map, tell one way the land will change during the second half of her trip.
Answers: 1

Chemistry, 22.06.2019 01:30
Sulfuric acid (a component of acid rain) reacts with limestone (calcium carbonate) to produce calcium sulfate and carbon dioxide. this damages buildings and statues made of limestone. which solution of sulfuric acid will damage these structures more quickly? a. 0.001% b. 0.005% c. 0.010% d. 0.015%
Answers: 3

Chemistry, 22.06.2019 04:30
There is a single path for electrons. the current decreases when additional resistors are added. the current will be the same in each resistor. these statements best describe a(n) circuit.
Answers: 3
You know the right answer?
It takes 38 mL of 0.75 M NaOH solution to completely neutralize 155 mL of a sulfuricacid solution (H...
Questions



Mathematics, 13.03.2021 21:50


Computers and Technology, 13.03.2021 21:50



Mathematics, 13.03.2021 21:50


Mathematics, 13.03.2021 21:50






History, 13.03.2021 21:50



Mathematics, 13.03.2021 21:50