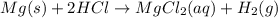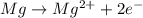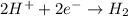Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
9. write the chemical equation for the following word equations. include symbols for physical states in the equation. a. solid zinc sulfide + oxygen gas -> solid zinc oxide + sulfur dioxide gas b. aqueous hydrochloric acid + aqueous barium hydroxide -> aqueous barium chloride + water
Answers: 1

Chemistry, 22.06.2019 14:30
How do temperature and salinity affect deepwater currents? as temperatures and salinity levels of water increase, the water rises to the surface where it creates currents as it moves to colder regions. they create changes in wind direction, moving denser water in the same direction as the wind and causing the deepwater circulation patterns found in the ocean. they equalize the forces on undersea currents caused by the coriolis effect as they replace more dense water with less dense water. they create density differences that cause dense deepwater currents to flow toward the equator where they displace less dense, warmer water above them.
Answers: 2


Chemistry, 22.06.2019 16:40
Identify the lewis acid in this balanced equation: ag+ + 2nh3 -> ag(nh3)2+a. ag+b. nh3c. ag(nh3)2+
Answers: 1
You know the right answer?
Well, we might as well try a similar question. Magnesium metal reacts with hydrochloric acid to prod...
Questions

Chemistry, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30

English, 29.10.2020 18:30


Chemistry, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30

Chemistry, 29.10.2020 18:30


Mathematics, 29.10.2020 18:30

Arts, 29.10.2020 18:30


English, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30


Arts, 29.10.2020 18:30


History, 29.10.2020 18:30

Arts, 29.10.2020 18:30

Mathematics, 29.10.2020 18:30

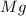 is being oxidized.
is being oxidized.