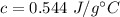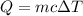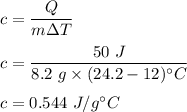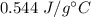Chemistry, 04.11.2020 18:40 abdullaketbi71
It takes 50.0 J to raise the temperature of an 8.20 g piece of unknown metal from 13.0∘C to 24.2 ∘C. What is the specific heat for the metal

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 11:50
Acompound has a molecular weight of 12.124 atomic mass units and the empirical formula c3h40. what is the molecular formula of the compound?
Answers: 3

Chemistry, 22.06.2019 12:30
Sodium sulfate dissolves as follows: na2so4(s) → 2na+(aq) + so42- (aq). how many moles of na2so4 are required to make 1.0 l of solution in which the na concentration is 0.10 m?
Answers: 2

Chemistry, 22.06.2019 12:30
The melting point of sulfur is 115 °c and its boiling point is 445 °c. what state would sulfur be in at 200 °c?
Answers: 1

Chemistry, 22.06.2019 14:00
In the space, show a correct numerical setup for calculating the number of moles of co2 present in 11 grams of co2
Answers: 1
You know the right answer?
It takes 50.0 J to raise the temperature of an 8.20 g piece of unknown metal from 13.0∘C to 24.2 ∘C....
Questions

World Languages, 27.09.2020 01:01





English, 27.09.2020 01:01

Biology, 27.09.2020 01:01



Arts, 27.09.2020 01:01


Mathematics, 27.09.2020 01:01


Advanced Placement (AP), 27.09.2020 01:01


Mathematics, 27.09.2020 01:01


Chemistry, 27.09.2020 01:01