
To prevent air from interacting with highly reactive chemicals, noble gases are placed over the chemicals to act as inert “blanketing” gases. A chemical engineer places a mixture of noble gases consisting of 5.50 g of He, 15.0 g of Ne, and 35.0 g of Kr in a piston-cylinder assembly at STP. Calculate the Partial Pressure per Gas

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 18:10
Using complete sentences, explain how to predict the products and balance the reaction between sulfuric acid and potassium hydroxide.
Answers: 1

Chemistry, 21.06.2019 21:00
Solar energy is energy from the sun that is converted into thermal or energy. a. nuclear b. mechanical c. electrical d. chemical
Answers: 2

Chemistry, 22.06.2019 01:00
The diagram shows the positions of the sun, moon and earth during spring tides, when the high tides are at their highest and low tides at their lowest. what is it about these positions that causes these high and low tides?
Answers: 3

Chemistry, 22.06.2019 04:00
4. absorption has the highest risk of overdose due to increased potency. a. rectal b. oral c. transdermal d. intranasal
Answers: 2
You know the right answer?
To prevent air from interacting with highly reactive chemicals, noble gases are placed over the chem...
Questions


History, 09.01.2022 01:00


Mathematics, 09.01.2022 01:00


English, 09.01.2022 01:00

Mathematics, 09.01.2022 01:00

Mathematics, 09.01.2022 01:10

Mathematics, 09.01.2022 01:10

Chemistry, 09.01.2022 01:10

SAT, 09.01.2022 01:10





English, 09.01.2022 01:10

Physics, 09.01.2022 01:10

Chemistry, 09.01.2022 01:10

Arts, 09.01.2022 01:10

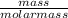
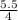 = 1.375mole
= 1.375mole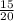 = 0.75mole
= 0.75mole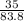 = 0.42mole
= 0.42mole 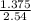 x 1atm = 0.54atm
x 1atm = 0.54atm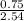 x 1atm = 0.29atm
x 1atm = 0.29atm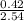 x 1atm = 0.17atm
x 1atm = 0.17atm

