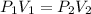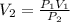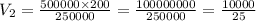Chemistry, 04.11.2020 02:40 eddsworldfrantic
The pressure is changed from 500 kPa to 250 kPa. What would you expect the new volume to be if the initial volume is 200 mL?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 16:00
Use the table to identify the phase and phase changes of the elements under the given conditions. write the name of the substance, phase, or phase change. when the temperature in a room increases from 25°c to 33°c, changes from a solid to a liquid. in a lab, methane and nitrogen are cooled from -170°c to -200°c. the methane freezes and the nitrogen . when gold is heated to 2,856°c it changes from a liquid to a .
Answers: 2



Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 1
You know the right answer?
The pressure is changed from 500 kPa to 250 kPa. What would you expect the new volume to be if the i...
Questions




Mathematics, 05.07.2019 22:30







Mathematics, 05.07.2019 22:30

Mathematics, 05.07.2019 22:30



Mathematics, 05.07.2019 22:30


Mathematics, 05.07.2019 22:30

English, 05.07.2019 22:30

Mathematics, 05.07.2019 22:30