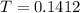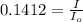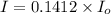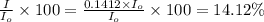Ireally need on this question!
the spectrophotometer really measures the percent of light t...

Chemistry, 30.01.2020 22:50 expeditionofsin
Ireally need on this question!
the spectrophotometer really measures the percent of light that is transmitted through the solution. the instrument then converts %t (transmittance) into absorbance by using the equation you determined in the pre-lab section. if the absorbance of a sample is 0.85, what is the percent light transmitted through the colored sample at this collected wavelength?
we only used the spectrophotometer to measure the absorbance and never got any %t values. so i have to convert absorbance to transmittance. how do i do this with the given absorbance of 0.85?

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 03:20
Which type of substance ionizes partially and gives off hydrogen ions when dissolved in water? a. strong acid b. strong base c. weak acid d. weak base
Answers: 1

Chemistry, 22.06.2019 10:00
How many grams of co(g) are there in 74.5 ml of the gas at 0.933 atm and 30o c?
Answers: 1

Chemistry, 22.06.2019 10:00
What is the atomic mass of an atom that has 6 protons, 6 neutrons, and 6 electrons? a) 6 b) 8 c) + 1 d) 12 e) 18
Answers: 1
You know the right answer?
Questions

Mathematics, 05.05.2021 22:20





Mathematics, 05.05.2021 22:20


Mathematics, 05.05.2021 22:20




Computers and Technology, 05.05.2021 22:20


Biology, 05.05.2021 22:20

Mathematics, 05.05.2021 22:20



Mathematics, 05.05.2021 22:20


![A=\log \frac{I_o}{I}=-\log[T]](/tpl/images/0487/5067/baad9.png)
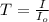
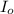 = incident light
= incident light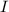 = transmitted light
= transmitted light![0.85=-\log[T]](/tpl/images/0487/5067/bf193.png)